Important: If there is problem in download, see Home page - How to download -.
Please report links do not work by writing a comment below or sending a message by Contact page.
Please report links do not work by writing a comment below or sending a message by Contact page.
هــام: اذا كان هناك مشكل في التحميل، شاهد الصفحة الرئيسية -كيفية التحميل-.
الرجاء الابلاغ عن روابط لا تعمل عن طريق كتابة التعليق أدناه أو إرسال رسالة عن طريق صفحة اتصل بنا.
الرجاء الابلاغ عن روابط لا تعمل عن طريق كتابة التعليق أدناه أو إرسال رسالة عن طريق صفحة اتصل بنا.
To download from FilesIn: User IDMAN Please desable It
للتحميل من FilesIn : مستعملي IDMAN يفضل تعطيله
------------------------------------
1- Softwares
In construction
2- Learning by Simulations
To be continued
5- Overlapping Chromatographic Peaks
In chromatography a common problem is the correct quantification of
overlapping peaks. Given that the two peaks are both at the baseline of
the chromatogram, the separation of the two peaks (and the subsequent
quantification) is performed by selecting the minimum between the two
peaks as the separation line. While this approach is quite reliable if
the peaks have the same height, it becomes more and more prone to
considerable errors if the heights of the two peaks do not match (which
is the common case).
This program simulates the quantification error made when dealing with
chromatographics peaks of unequal height. The user may experiment with
different peak positions, heights and peak widths.
Download:
English version http://aa.vg/ljtihlkbm4fr [323kB]
German version http://aa.vg/5h37z1jqo4hr [324 kB]
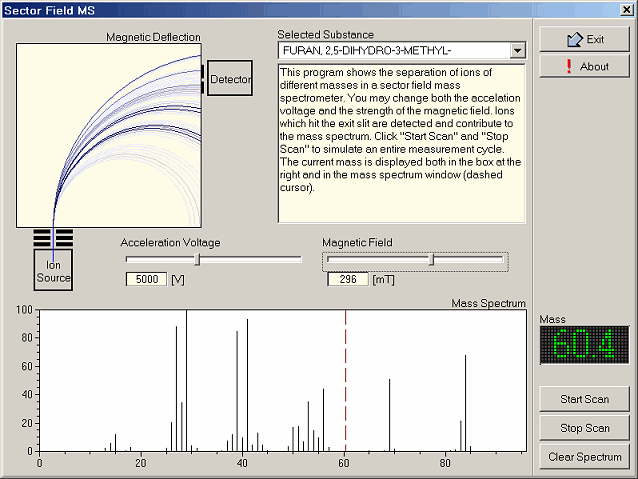
4- Sector Field MS
The magnetic sector field is - among other principles such as electric
quadrupole fields, or flight time measurements - one of the most often
used principles for commerical mass spectrometers. The principle is easy
to understand: a beam of accelerated ions is directed into a magnetic
field whose orientation is perpenticular to the beam. The magnetic field
thus forces the charged particles on different circular trajectories,
whose radii depend on the strength of the magnetic field, the
accelerating voltage, and on the mass of the particular ion.
Keeping all but the magnetic field constant results in a separation of
the ions according to their mass. Since the detector has a narrow entry
split, it "sees" only a small fraction of the entire ion beam fan, i.e.
those ions which travel along the circular trajectory connecting both
the exit slit of the ion source and the entry slit of the detector. All
other ions collide with the inner wall of the evacuated separation
system (a small rectangular tube of non-magnetic material).
The program ms_sectorfield simulates the separation of ions of
different masses in a magnetic sector field. The user can adjust both
the accelerating voltage and the magnetic field strength, and select
from a small number of substances, whose mass spectra have been provided
by NIST (courtesy S. Stein of NIST).
Download:
English version http://aa.vg/iz7oj31slij5 [387 kB]
German version http://aa.vg/uafqqzczjd66 [387 kB]
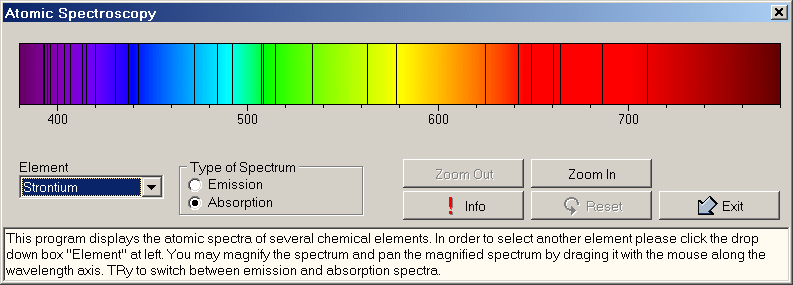
3- Atomic Spectra
Atomic spectra may be used to quantitatively determine more than 70 chemical elements. In order to be able to detect the atomic spectrum, the atoms or ions have to be separated from one another, i.e. the atoms have to be in a gaseous state. This can be achieved by massively heating the substances to be determined (typically the temperature is several thousand degrees). When the gas heats up, it emits light of various characteristic wavelengths. For hydrogen the resulting spectrum obeys a very simple relation:
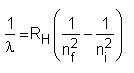
RH = 1.097 107 m-1.
107 m-1.
Apart from chemical analysis atomic spectroscopy is of considerable
importance for the investigation of stars, looking for elemental
compositions which could enable life in some form. The interest in
atomic emission and absorption lines can be traced back to the late 18th and the early 19th Century when Fraunhofer discovered dark lines in the spectrum of the sun light. 107 m-1.
107 m-1.
f you would like to experiment with spectral lines of selected elements, you can use the program AtomSpec which allows the user to select from several chemical elements. The spectrum may be zoomed to view details of the atomic spectral lines.
Download:
English version http://aa.vg/615593albvgh [266 kB]
German version http://aa.vg/hr3n6bwkylxx [266 kB]
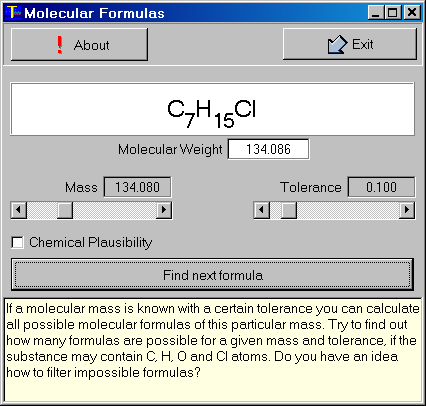
2- Molecular Formulas
To explain this, let's have a look at a very simple example: suppose we know that our substance has a molecular weight of 28. A quick calculation (considering only the nominal masses of the most abundant isotopes of carbon, hydrogen, oxygen, and nitrogen - 12, 1, 16, and 14, respectively) shows that there are three substances which all have the same molecular mass of 28: C2H4 (ethene), CO (carbon monoxide) and N2 (nitrogen gas).
Now, the important point is not to forget that the nominal masses are just for convenience. The actual masses of the four elements are:
1H = 1.007825037
16O = 15.99491464
14N = 14.003074
- C2H4 = 28.031300148
- N2 = 28.006148
- CO = 27.99491464
This simple example shows the principle of finding the atomic composition by means of mass spectrometry. You simply have to determin the mass with an accuracy high enough to reduce the number of possible candidates to a single molecule. Of course, in practice the molecular masses of interest are much higher and the number of possible candidates increases exponentially if we do not take countermeasures, such as a chemical plausibility check.
The program MolForm allows to calculate all possible molecular formulas of a particular mass. The user may set the molecular mass and its tolerance (accuracy). This example shows the possible formulas for molecules containing C, H, O, N, and Cl atoms.
Download:
English version http://aa.vg/vwod418b55lv [266 kB]
German version http://aa.vg/33gbd30pv3jq [266 kB]
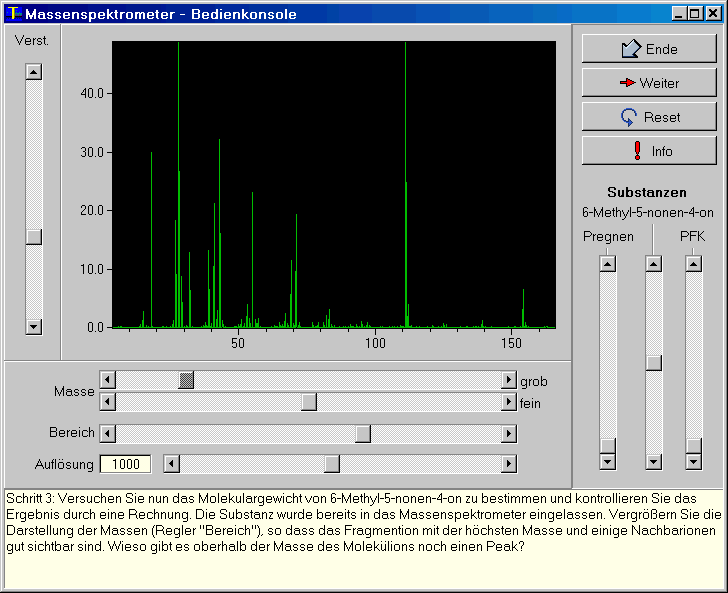
1- Console of a Mass Spectrometer
A mass spectrometer is a device which can perform accurate chemical analysis (both quantitative and qualitative). Although there are several different technical implementations (sector field, quadrupole, time of flight) all of these devices are based on the same idea: the molecules under investigation are first broken up and then the type and the amount of fragments are investigated. The kind of fragments provides information on the chemical structure of the original molecule, the amount of fragmented ions allows to determine the quantities. Regardless of the actual ionisation and separation technique all kinds of mass spectrometers eventually yield a mass spectrum, which is a line spectrum showing the fragment mass on the x-axis and the number of generated ions on the y-axis.
This program ms_scope simulates the console of a mass
spectrometer. The user may experiment with different resolutions and
mass ranges. Ambient air, pregnene, 6-methyl-5-nonen-4-one and
perfluorokerosene may be put into the simulated mass spectrometer.
Download:
3- Books
English
To be continued
___________________________________________________________________________
6- Macrocyclic Chemistry: New Research Developments
Daniel W. Fitzpatrick, Henry J. Ulrich, "Macrocyclic Chemistry: New Research Developments"
English | 2010 | ISBN: 1608768961 | 517 pages | PDF | 6,9 MB
English | 2010 | ISBN: 1608768961 | 517 pages | PDF | 6,9 MB
A macrocycle is, as defined by IUPAC, 'a cyclic macromolecule or a macromolecular cyclic portion of a molecule'. In the chemical literature, organic chemists may consider any molecule containing a ring of seven or more atoms to be a macrocyle. Co-ordination chemists generally define a macrocycle more narrowly as a cyclic molecule with three or more potential donor atoms that can co-ordinate to a metal centre. This book brings together the latest research results from around the world.

or
___________________________________________________________________________
5- Workbook for Organic Chemistry
Jerry Jenkins, "Workbook for Organic Chemistry"
English | 2009 | ISBN: 1429247584 | 512 pages | PDF | 3,9 MB
English | 2009 | ISBN: 1429247584 | 512 pages | PDF | 3,9 MB
Jerry Jenkins’ extensive workbook provides approximately 80 problems per topic with full worked out solutions. The perfect aid for students in need of more problem-solving, the Workbook for Organic Chemistry can be paired with any organic chemistry text on the market. For instructors interested in online homework, W.H. Freeman has also placed these problems in WebAssign.

or
4- Inspection,
Prevention, Control, and Repair of Corrosion
Avionics Equipement
Link: http://www.filesin.com/4023D216773/download.html
________________________________________________________________________________
3- Handbook of chemistry
Link: http://www.restfile.com/zwjtdss7w9ah/chem-v1.pdf.html
________________________________________________________________________________
2- The Chemistry of Paints and Painting
is a free textbook on
chemical aspects of painting. This ebook is based on the printed copy
of a book written by Sir Arthur H. Church in 1915.
approx. 180 pages, 10 figures
2.2 MB
1- General Chemistry
is a free textbook on the fundamentals of chemistry
This book is currently under construction.
2.2 MB
________________________________________________________________________________
عــربـــــــــــيــة
يــتــبــع
________________________________________________________________________________
8- السلامة_في_المختبرات_الكيميائية
http://www.restfile.com/matp7ej6dntc/السلامة_في_المختبرات_الكيميائية.pdf.html
_________________________________________________________________________________
_________________________________________________________________________________
_________________________________________________________________________________
________________________________________________________________________________
http://www.restfile.com/jel5sk529lue...Theor.pdf.htm
8- السلامة_في_المختبرات_الكيميائية
http://www.restfile.com/matp7ej6dntc/السلامة_في_المختبرات_الكيميائية.pdf.html
_________________________________________________________________________________
7- أساسيات_الكيمياء_الفيزيائية-عملي
http://www.restfile.com/01bv85tx0mgv/أساسيات_الكيمياء_الفيزيائية-عملي.pdf.html_________________________________________________________________________________
6- أساسيات_الكيمياء_الفيزيائية-نظري
http://www.restfile.com/zz8afo8wwvhp/أساسيات_الكيمياء_الفيزيائية-نظري.pdf.html_________________________________________________________________________________
5- أساسيات_الكيمياء_العضوية-عملي
________________________________________________________________________________
4- أساسيات_الكيمياء_العضوية_-_نظري
________________________________________________________________________________
3- الكيمياء_العامة
http://www.restfile.com/58gk4qlb2zge/الكيمياء_العامة.pdf.html
_________________________________________________________________________________
2- أساسيات الكيمياء التحليلية - عملي.pdf
http://www.restfile.com/2k5evmviluup/Basic_Anal_Chem_Pratique.pdf.html
3- الكيمياء_العامة
http://www.restfile.com/58gk4qlb2zge/الكيمياء_العامة.pdf.html
_________________________________________________________________________________
2- أساسيات الكيمياء التحليلية - عملي.pdf
http://www.restfile.com/2k5evmviluup/Basic_Anal_Chem_Pratique.pdf.html
_________________________________________________________________________________
1- أساسيات الكيمياء التحليلية - نظري.pdfhttp://www.restfile.com/jel5sk529lue...Theor.pdf.htm